After ICGC ARGO’s initial strategy meeting in 2018, and further planning in 2019, the third annual meeting was held online in June and it was a showcase of data in action.
This past year, ICGC ARGO members have made great strides in assembling core infrastructure, expertise and workflows to underpin the consortium’s ambition to deliver a million patient-years of precision oncology knowledge to the world.
Despite the circumstances moving ICGC ARGO’s third annual meeting online, new and established working groups and member programs shared how they are forging ahead on the path to precision oncology.
As of June 2020, ICGC ARGO has 58,300 committed donors from 25 programs in 13 countries with a greater focus on metastatic cancers and clinical trials.
“But we don’t learn from as many patients as we should,” said Andrew Biankin, Executive Director based at the University of Glasgow, reflecting on ICGC ARGO’s central purpose to open the meeting.
The newly formed Pathology Working Group outlined its vision of integrating pathology expertise in all ICGC ARGO projects to provide in-depth tissue analyses beyond diagnosis. The group is looking to incorporate state-of-the-art approaches, such as structured synoptic reporting, and to leverage the wealth of digital imaging data now available in pathology.
“Doing this will not be easy,” said Mark Rubin, from the University of Bern, who leads the Pathology Working Group and the ICGC ARGO Swiss Precision Oncology Program. “But we think this would be the highest level that we could achieve for integrating pathology data into ICGC ARGO.”
Likewise, the committee overseeing ICGC ARGO’s community outreach strengthened their ambition to be a leader in patient engagement, forming the Patient and Public Involvement and Engagement (PPIE) Working Group. Given the project is inherently a patient focused initiative; the group committed to harnessing the invaluable expertise of patients and carers to inform activities. Patient involvement in consent practices, data sharing policies and the accessibility of ICGC research are early priorities underway.
In headline news, the Data Coordination Centre’s (DCC) Christina Yung, based at the Ontario Institute for Cancer Research (OICR), announced that the ICGC ARGO data platform has launched and it was on full show during the meeting.
“This is where the rubber meets the road,” said Lincoln Stein, who leads the Data Coordination and Management Working Group.
The centralised platform will harmonise ICGC ARGO data submissions, curation and distribution around the world – shaped by learnings from the recently published Pan-Cancer analysis which brought together more than 2,600 whole cancer genomes.
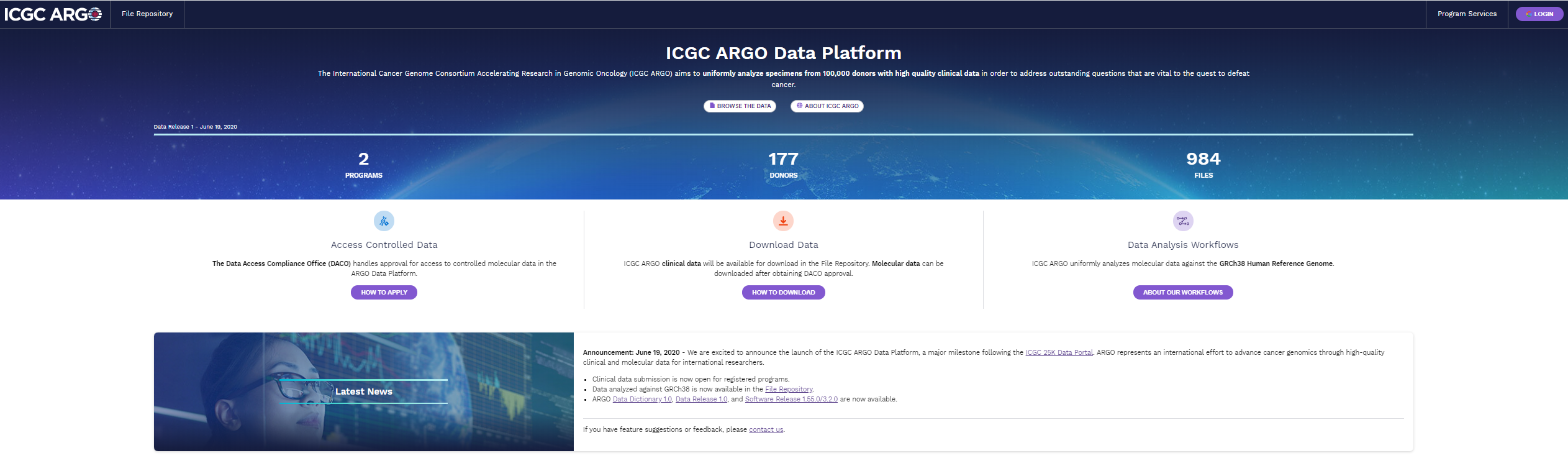
Built on foundational software already installed at the three regional ICGC ARGO data processing centres, the data platform is now ready to accept sample registrations and submissions of core clinical data.
Molecular data submissions will be accepted later this year, and the DCC is working towards automated workflows that initiate sequence alignment and analysis on receipt of all required data.
Researchers can already access via the data platform the first ICGC ARGO data release of nearly 180 genomes with almost 1,000 data files from two programs. The genomes, collected for the ICGC 25K Initiative and repurposed to stress test the new data system, have been lifted to the latest ICGC ARGO clinical and molecular standards.
Central to this effort is the ICGC ARGO ‘data dictionary’, developed in consultation with representatives from each research program.
The data dictionary will standardise the quality of every ICGC ARGO sample. It defines the minimal set of clinical and tissue-specific fields required for a patient or sample to be accepted as an ICGC ARGO case, and all data will be validated against it upon submission.
As bioinformatician Hardeep Nahal-Bose from the OICR’s DCC team explained, the dictionary and larger data model have been designed to support longitudinal data collection which will enable researchers to ask questions that might otherwise require a dedicated, stand-alone study to resolve.
But of course, real-world data doesn’t always fit neatly into standard codes as Amber Johns of the Tissue and Clinical Annotation Working Group showed with a walk-through of clinical data files curated for the new ICGC ARGO data platform from the Australian ICGC program.
This means all ICGC ARGO contributors will need to double down on their commitment to assembling quality datasets with the granularity to answer key clinical questions and the systems to truly accelerate cancer research in a rapid and responsible way.
“Transforming patient clinical data and their treatment journeys into analysable, actionable information requires meticulous, skilled data curation,” Johns said. “But hard work pays off.”